Chemquest 42 moles and reactions – In the realm of chemistry, Chemquest 42: Moles and Reactions takes center stage, inviting us to delve into the intricacies of chemical transformations. This guide unravels the concepts of moles and chemical reactions, equipping us with the knowledge to navigate the complexities of stoichiometry and predict the outcomes of reactions.
As we embark on this journey, we will explore the fundamental principles of mole calculations, the art of balancing chemical equations, and the significance of stoichiometry in understanding the quantitative relationships between reactants and products. Along the way, we will encounter real-world applications that showcase the practical relevance of these concepts.
Introduction: Chemquest 42 Moles And Reactions
In chemistry, understanding the concept of moles is crucial for accurate calculations and predictions. A mole is the standard unit of measurement for the amount of a substance, representing a specific number of atoms, molecules, or ions. It serves as a bridge between the macroscopic and microscopic scales, allowing chemists to relate the mass and volume of a substance to its chemical composition.
Chemical reactions involve the rearrangement of atoms or molecules to form new substances. Understanding moles enables us to determine the stoichiometry of reactions, ensuring that reactants are present in the correct proportions to produce the desired products. By understanding moles, chemists can predict the quantities of reactants and products involved in a reaction, facilitating accurate experimentation and the optimization of chemical processes.
Importance of Moles in Chemistry
- Quantitative Analysis:Moles allow for the determination of the precise amount of a substance present in a sample, facilitating quantitative analysis and the determination of unknown concentrations.
- Stoichiometry:Understanding moles is essential for balancing chemical equations and predicting the quantities of reactants and products involved in a reaction.
- Molar Mass:The molar mass of a substance, expressed in grams per mole, provides a direct link between its mass and the number of moles present, enabling the conversion between mass and moles.
- Gas Calculations:Moles are used to determine the volume of gases under specific conditions, using the ideal gas law, PV = nRT.
- Solution Chemistry:Moles are crucial for understanding solution concentrations, such as molarity, molality, and mass percentage.
Mole Calculations
In chemistry, the mole is a fundamental unit of measurement used to quantify the amount of a substance. It is defined as the amount of substance that contains exactly 6.022 × 10 23elementary entities, such as atoms, molecules, or ions.
The mole concept is essential for understanding stoichiometry, which involves the quantitative relationships between reactants and products in chemical reactions.
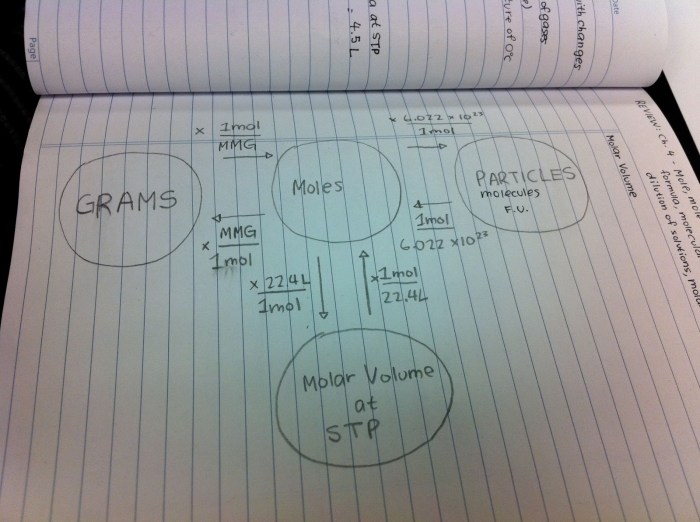
Converting Between Mass, Moles, and Number of Particles
The following formulas can be used to convert between mass, moles, and number of particles:
- Mass (g) = Moles (mol) × Molar Mass (g/mol)
- Moles (mol) = Mass (g) / Molar Mass (g/mol)
- Number of Particles = Moles (mol) × Avogadro’s Number (6.022 × 10 23particles/mol)
Examples of Mole Calculations
Example 1:
Calculate the number of moles in 25.0 g of NaCl.
Solution:
Molar Mass of NaCl = 58.44 g/mol
Moles of NaCl = Mass / Molar Mass
Moles of NaCl = 25.0 g / 58.44 g/mol
Moles of NaCl = 0.428 mol
Example 2:
Calculate the mass of 0.500 mol of Fe 2O 3.
Solution:
Molar Mass of Fe 2O 3= 159.69 g/mol
Mass of Fe 2O 3= Moles × Molar Mass
Mass of Fe 2O 3= 0.500 mol × 159.69 g/mol
Mass of Fe 2O 3= 79.85 g
Balancing Chemical Equations
Balancing chemical equations is a crucial step in stoichiometry, as it ensures that the number of atoms of each element on the reactants’ side of the equation equals the number of atoms of that element on the products’ side. This adherence to the law of conservation of mass is essential for accurate chemical calculations.
To balance chemical equations, follow these steps:
Balancing by Inspection, Chemquest 42 moles and reactions
Begin by identifying the unbalanced equation. Then, inspect the equation and try to balance it by adjusting the coefficients in front of each chemical formula. The coefficients represent the number of molecules or moles of each reactant and product. Start with the most complex molecule and work your way down.
For example, consider the unbalanced equation: 2C2H6 + 7O2 → 4CO2 + 6H2O. To balance this equation, adjust the coefficients as follows:
- Balance the carbons: 2C2H6 → 4C
- Balance the hydrogens: 2C2H6 → 6H
- Balance the oxygens: 7O2 → 14O
The balanced equation is: 2C2H6 + 7O2 → 4CO2 + 6H2O.
Balancing by Half-Reaction Method
When balancing more complex equations, the half-reaction method is often more effective. This method involves balancing the oxidation and reduction half-reactions separately before combining them to form the overall balanced equation.
- Identify the oxidation and reduction half-reactions.
- Balance each half-reaction in terms of mass and charge.
- Multiply the half-reactions by appropriate factors to balance the number of electrons transferred.
- Combine the balanced half-reactions to form the overall balanced equation.
For example, consider the unbalanced equation: Fe + HCl → FeCl2 + H 2. The oxidation half-reaction is Fe → Fe2+ + 2e-, and the reduction half-reaction is 2H+ + 2e- → H 2. Balancing the half-reactions and multiplying them by appropriate factors, we get:
- Fe → Fe2+ + 2e-
- 2H+ + 2e- → H2
Multiplying the oxidation half-reaction by 2 and combining it with the reduction half-reaction, we obtain the balanced equation: Fe + 2HCl → FeCl2 + H2.
Stoichiometry

Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It is a fundamental tool for predicting the outcome of chemical reactions and for designing experiments.
Stoichiometry can be used to solve a variety of problems, including:
- Predicting the products of a reaction
- Calculating the amount of reactants or products that are needed for a reaction
- Determining the limiting reactant in a reaction
Using Stoichiometry to Predict the Products of a Reaction
To use stoichiometry to predict the products of a reaction, you need to know the balanced chemical equation for the reaction. The balanced chemical equation will tell you the mole ratios of the reactants and products.
For example, the following balanced chemical equation shows the reaction between hydrogen and oxygen to form water:
“`
H2+ O 2→ 2H 2O
“`
This equation tells us that 2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water.
To predict the products of a reaction, you can use the mole ratios in the balanced chemical equation. For example, if you want to predict the products of the reaction between 1 mole of hydrogen and 1 mole of oxygen, you can use the following steps:
- Write the balanced chemical equation for the reaction:
- Determine the mole ratio of the reactants and products:
- Use the mole ratio to predict the products of the reaction:
- Calculate the moles of each reactant using their given masses and molar masses.
- Determine the stoichiometric ratio of the reactants from the balanced chemical equation.
- Divide the moles of each reactant by its stoichiometric coefficient.
- The reactant with the smallest result is the limiting reactant.
- Chemical Manufacturing:Stoichiometry helps determine the precise amounts of reactants and products in chemical reactions, optimizing production efficiency and minimizing waste.
- Pharmaceuticals:Accurate stoichiometry is essential for drug synthesis, ensuring the correct dosage and effectiveness of medications.
- Environmental Engineering:Stoichiometry aids in understanding and controlling chemical reactions in environmental processes, such as wastewater treatment and pollution remediation.
“`
H2+ O 2→ 2H 2O
“`
“`
moles H2: 1 mole O 2: 2 moles H 2O
“`
“`
mole H2+ 1 mole O 2→ 1 mole H 2O
“`
Therefore, the products of the reaction between 1 mole of hydrogen and 1 mole of oxygen are 1 mole of water.
Limiting Reactants
In a chemical reaction, the limiting reactant is the reactant that is completely consumed and determines the maximum amount of product that can be formed. The other reactants are present in excess and are not completely consumed.
To identify the limiting reactant, compare the mole ratio of the reactants to their stoichiometric ratio. The reactant with the smallest mole ratio relative to its stoichiometric ratio is the limiting reactant.
Identifying Limiting Reactants
Percent Yield
Percent yield is a measure of the efficiency of a chemical reaction. It is calculated by dividing the actual yield of a reaction by the theoretical yield, and then multiplying by 100%. The theoretical yield is the amount of product that would be produced if the reaction went to completion, assuming 100% efficiency.
The percent yield can be affected by a number of factors, including the purity of the reactants, the reaction conditions, and the presence of side reactions.
Calculating Percent Yield
The percent yield can be calculated using the following formula:
Percent yield = (Actual yield / Theoretical yield) x 100%
For example, if a reaction produces 10 g of product and the theoretical yield is 15 g, the percent yield would be:
Percent yield = (10 g / 15 g) x 100% = 66.67%
Applications of Moles and Reactions

Understanding moles and reactions is crucial in various scientific and industrial fields. Stoichiometry plays a vital role in ensuring efficient and accurate processes in industries such as chemical manufacturing, pharmaceuticals, and environmental engineering.
Importance of Stoichiometry in Industries
Query Resolution
What are moles?
A mole is the SI unit of amount of substance, defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
Why is it important to understand moles in chemistry?
Moles provide a convenient way to express the amount of a substance in terms of its mass or number of particles, allowing us to perform stoichiometric calculations and predict the outcomes of chemical reactions.
How do I balance chemical equations?
Balancing chemical equations involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation.
What is stoichiometry?
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions.
How can I identify the limiting reactant in a reaction?
The limiting reactant is the reactant that is completely consumed in a reaction, limiting the amount of product that can be formed.