The half life of cesium 137 is 30 years – The half-life of cesium-137 is 30 years, making it a significant radioactive isotope with applications in various fields and potential environmental concerns. This comprehensive guide delves into the properties, applications, environmental impact, and safety considerations of cesium-137, providing a thorough understanding of its importance and implications.
Cesium-137, a radioactive isotope with an atomic number of 55 and an atomic mass of 137, is a product of nuclear fission and is widely used in medical and industrial applications. Its unique properties and relatively long half-life make it a valuable tool in various fields while also posing potential risks that require careful management and disposal.
Properties of Cesium-137
Cesium-137 is a radioactive isotope of cesium with an atomic number of 55 and an atomic mass of 136.907. It is a member of the alkali metal group and has a chemical symbol of Cs. Cesium-137 is produced through nuclear reactions, such as neutron activation of stable cesium isotopes, and is a significant contributor to radioactive contamination in the environment.
Significance of Cesium-137 as a Radioactive Isotope
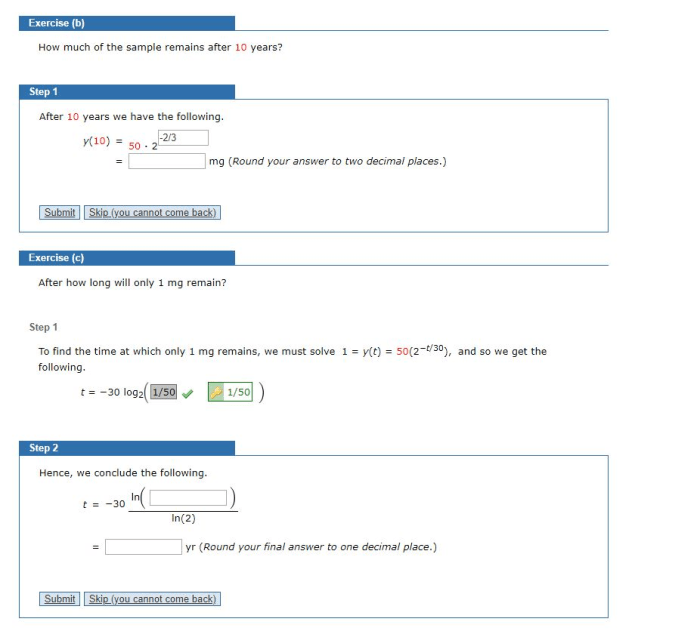
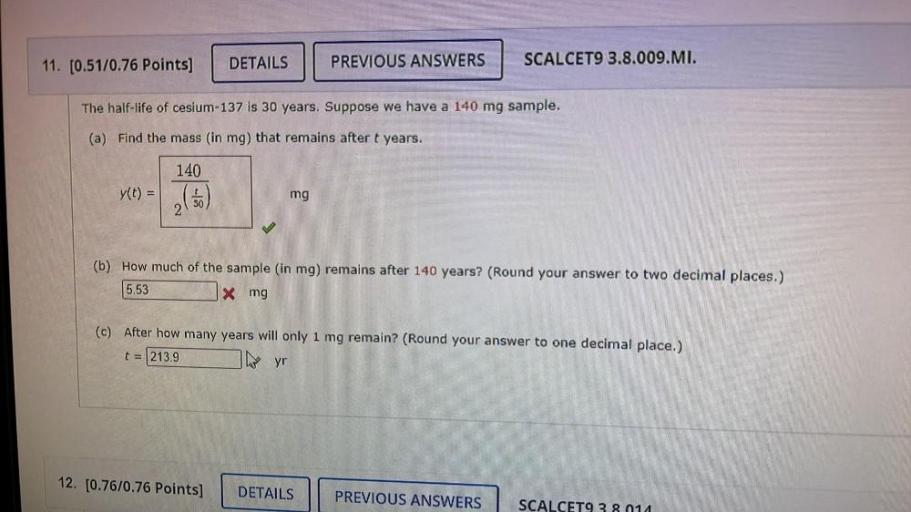
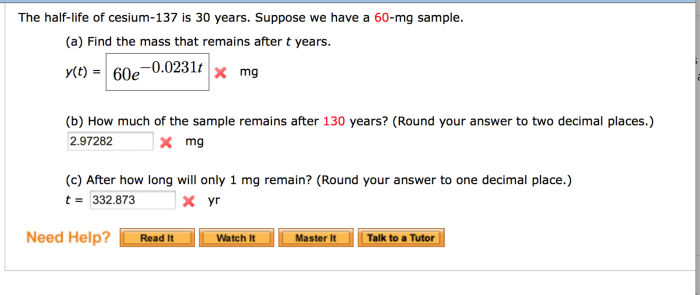
Cesium-137 is a radioactive isotope with a relatively long half-life of 30 years. This means that it takes 30 years for half of the Cesium-137 atoms in a sample to decay. The decay of Cesium-137 releases high-energy gamma rays, making it a potential hazard to human health and the environment.
Half-Life of Cesium-137
Concept of Half-Life in Radioactive Decay
Half-life is a fundamental concept in radioactive decay. It represents the amount of time it takes for half of the atoms in a radioactive sample to decay. The half-life is constant for a given isotope and is independent of the initial amount of the isotope present.
Significance of the 30-Year Half-Life of Cesium-137, The half life of cesium 137 is 30 years
The 30-year half-life of Cesium-137 is significant because it determines the rate at which the isotope decays and the potential risks associated with its presence in the environment. The long half-life means that Cesium-137 can persist in the environment for a considerable amount of time, posing potential risks to human health and ecosystems.
Calculating the Amount of Cesium-137 Remaining
To calculate the amount of Cesium-137 remaining after a given time period, the following formula can be used:
Amount remaining = Initial amount
(1/2)^(time elapsed / half-life)
For example, after 60 years, the amount of Cesium-137 remaining would be:
Amount remaining = Initial amount
- (1/2)^(60 / 30) = Initial amount
- 1/4
After 90 years, the amount of Cesium-137 remaining would be:
Amount remaining = Initial amount
- (1/2)^(90 / 30) = Initial amount
- 1/8
Commonly Asked Questions: The Half Life Of Cesium 137 Is 30 Years
What is the significance of the 30-year half-life of cesium-137?
The 30-year half-life means that it takes 30 years for half of the radioactive cesium-137 atoms in a sample to decay, making it a relatively long-lived radioactive isotope.
What are the potential environmental risks associated with cesium-137?
Cesium-137 can be released into the environment through nuclear accidents or incidents, posing risks to human health and ecosystems. It can accumulate in soil and water, potentially exposing humans and animals to radiation.
What safety precautions are necessary when handling cesium-137?
Handling cesium-137 requires proper training, protective equipment, and adherence to safety regulations. It is essential to minimize exposure to radiation and follow established guidelines for storage, transportation, and disposal.