Classify the statements about redox reactions as true or false. – In the realm of chemistry, redox reactions play a pivotal role in understanding electron transfer and chemical transformations. Classifying statements about redox reactions as true or false requires a comprehensive understanding of the underlying principles and criteria. This guide will delve into the intricacies of redox reactions, empowering you to make accurate classifications and unravel the mysteries of chemical processes.
Redox reactions involve the transfer of electrons between atoms or ions, leading to changes in their oxidation states. Oxidation refers to the loss of electrons, while reduction involves the gain of electrons. Understanding these fundamental concepts is crucial for classifying statements about redox reactions.
Redox Reactions
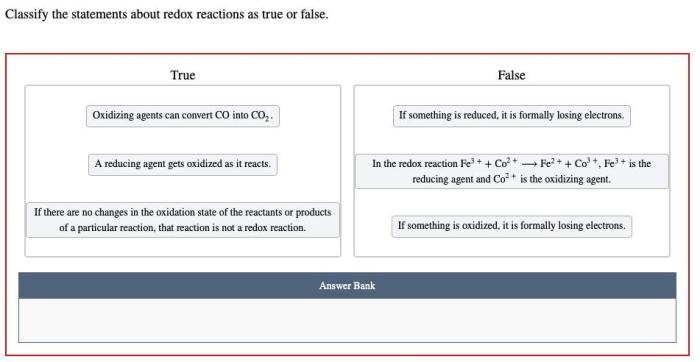
Redox reactions involve the transfer of electrons between atoms or ions, leading to changes in their oxidation states. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. Oxidizing agents facilitate oxidation by accepting electrons, while reducing agents facilitate reduction by donating electrons.
Classifying Statements about Redox Reactions
Classifying statements about redox reactions as true or false requires understanding the fundamental principles of redox reactions. True statements accurately describe the processes involved, while false statements contain errors or misconceptions. Accurately classifying statements helps in comprehending redox reactions and making informed predictions about their outcomes.
Criteria for Classification
Key criteria for classifying statements about redox reactions include:
- Chemical equations:Balanced chemical equations provide information about the reactants, products, and the changes in oxidation states that occur.
- Half-reactions:Dividing redox reactions into half-reactions (oxidation and reduction half-reactions) helps identify the species undergoing oxidation and reduction.
- Oxidation state analysis:Analyzing the change in oxidation states of reactants and products reveals whether oxidation or reduction has occurred.
Examples of Classification
| Statement | Classification | Explanation |
|---|---|---|
| In a redox reaction, the oxidizing agent undergoes reduction. | False | The oxidizing agent accepts electrons and undergoes reduction, while the reducing agent donates electrons and undergoes oxidation. |
| The oxidation number of an element can only increase in a redox reaction. | False | Oxidation numbers can increase (oxidation) or decrease (reduction) in redox reactions. |
| In a balanced redox reaction, the total number of electrons lost is equal to the total number of electrons gained. | True | Electron transfer must be balanced to maintain charge neutrality. |
Applications of Classification, Classify the statements about redox reactions as true or false.
Classifying statements about redox reactions has practical applications in various fields:
- Electrochemistry:Understanding redox reactions is crucial for designing and optimizing electrochemical cells, batteries, and fuel cells.
- Environmental chemistry:Redox reactions play a significant role in environmental processes such as water treatment, pollution control, and remediation.
- Analytical chemistry:Redox reactions are used in analytical techniques such as titrations and electrochemical sensors.
FAQ Insights: Classify The Statements About Redox Reactions As True Or False.
What are the key criteria for classifying redox reaction statements?
Chemical equations, half-reactions, and changes in oxidation states of reactants and products are essential criteria for accurate classification.
How can redox reaction classification aid in understanding chemical processes?
Classifying redox reactions helps identify the electron transfer pathways, predict reaction outcomes, and analyze the reactivity of chemical species.
What are some practical applications of redox reaction classification?
Redox reaction classification finds applications in electrochemistry, environmental chemistry, and various industrial processes, enabling the design and optimization of chemical systems.